TrialSummaries.com is the leading platform for sharing clinical trial summaries globally. Our sponsor-neutral platform hosts over 15,000 studies with 8,000 plain language summaries (PLS) in an average of 4 languages for access to over 6,500 PLS. We reach visitors from over 90 countries and facilitate over 14,000 downloads each year, bringing trial results to a vast and global audience.
Many regulatory bodies mandate sharing trial results with participants. Clear and accessible clinical trial summaries help patients understand complex scientific data, build trust between sponsors and participants, and ensure sponsors meet legal and ethical obligations. These summaries also educate participants about trial outcomes, fostering engagement and future involvement.
TrialSummaries.com offers a dedicated platform to streamline the distribution process, ensuring your well-crafted summaries reach the participants who made your research possible.
Trial Summaries by the numbers
90+
countries40,000
visitors annually15,000
studies8,000
with summaries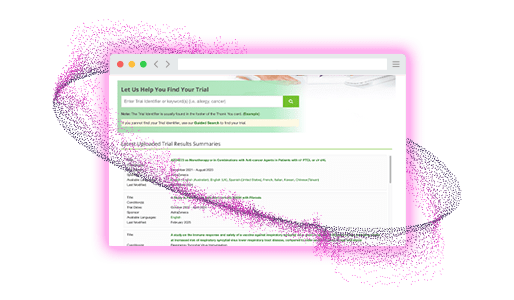
How it helps
TrialSummaries.com simplifies the distribution of plain language summaries through a centralized, accessible platform. Participants can subscribe to receive notifications for new summaries, while sponsors retain full control over content and publication timing. This digital approach reduces traditional printing and shipping constraints, ensuring consistent availability of study information to participants, and promotes transparency and trust with trial participants, the public, and regulatory authorities.
How it works
Create
Upload studies via multiple means
Create
- Upload studies via manual entry, ClinicalTrials.gov import, or automatic feed from TrialScope Disclose
- Maintain control through our secure administrative portal
Share
Distribute unique study URLs
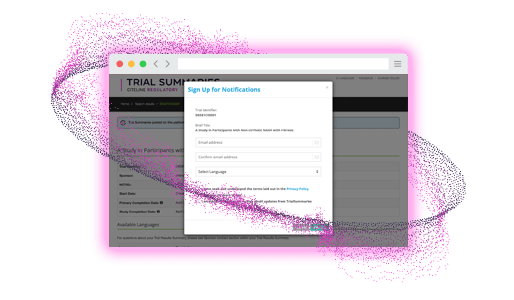
Subscribe
Participants sign up to be notified
Publish
Release summaries on your timeline
Notify
Automatic updates to participants
Track
Monitor engagement analytics
Create
- Upload studies via manual entry, ClinicalTrials.gov import, or automatic feed from TrialScope Disclose
- Maintain control through our secure administrative portal
Global Access Platform
- Mobile-responsive interface
- Multi-language support in over 45 languages
- Ability to post summaries in over 250 languages
Participant Engagement
- Guided search to help find trials easily
- Automated notifications for subscribed users when trial summaries are available
Sponsor Control Center
- Full control over study content
- Secure administrative portal
- Analytics and engagement tracking
The industry’s leading experts on clinical trial disclosure inform our TrialScope Solutions, Trial Summaries and Disclosure Services.



Contact us today to learn how we can support your trial summary needs.